Business
A Complete Information of Global Unique Device Identification Database GUDID

Where can hospitals, clinicians, patients, researchers and interested stakeholders access information about a particular medical device? The Global Unique Device Identification Database (GUDID Services), administered by the FDA, serves as this database by providing a comprehensive reference catalog for each medical device with a Unique Device Identifier (UDI).
The database only includes information important to the identification of devices and does not include any information identifying a patient. All 64 data elements required by 21 CFR 830.310 (directing electronic records to be maintained or submitted to the FDA) are included.
Some of the key benefits is the ability to facilitate quick and accurate medical device identification, removing potential confusion about device use. High standards for accurate data to be submitted and ensuring product accuracy for downstream users is also another crucial benefit. Top-tier device manufacturers are pairing the UDI together with the digital thread for end-to-end traceability of their data.
History of GUDID
The FDA established the UDI system in 2013, requiring medical devices to include a device label in a machine and human-readable format. Within the UDI system, GUDID was created as a foundational database used to store medical device product information associated with the UDI. The database officially launched in December 2013, with the public access portal, AccessGUDID, available in May 2015.
Data submission requirements have been deciphered by the medical device risk class, with various deadlines for compliance. Class I medical devices were originally required to be submitted to the GUDID by September 24, 2020, however, unforeseen supply chain shortages from the pandemic delayed this submission requirement to September 24, 2022. See the timeline, below.
- September 24, 2014 – Class III devices
- September 24, 2015 – Implantable, life-supporting, or life-sustaining (I/LS/LS) devices
- September 24, 2016 – Class II devices
- September 24, 2022 – Class I devices
Who Needs to Submit Data to the GUDID?
Mandated by the FDA, medical device labelers are required to submit data records for medical devices marketed in the United States to the GUDID. The FDA Final Rule provides a thorough definition of a labeler. As defined, the labeler is the entity that applies or modifies the label and brings the device to market under its brand. Typically, this consists of the medical device manufacturer, however it may also be the specification developer, device distributor, reprocessor, repackager or relabeler. The regulatory or compliance team of a medical device company is usually responsible for determining if they constitute as the labeler, per FDA’s definition.
If more than one entity is involved in bringing a medical device product to the market, the labeler responsible for submitting product data must define this joint venture in a written agreement. Duplicate submissions should be avoided and failure to submit product data altogether may result in both entities being held accountable to the FDA.
Also Read: To know the best employee engagement software read the blog.
What Data Must Be Submitted?
A total of 64 data elements are required for submission, with 57 submitted by the labeler and 7 populated by the FDA. The FDA has provided a spreadsheet of data requirements. Note, only the Device Identifier (DI) portion of a UDI code is submitted to the GUDID, per FDA guidance.
The required specifications for GUDID submission embody all of the device information available in the UDI, including a detailed device description and version/model, Issuing Agency, labeler company’s name, address and DUNS code. Additional information is also required per submission, including some of the following:
Commercial distribution – Determines when a record would be publicly available on AccessGUDID, whether the device is in commercial distribution, and when the device is no longer offered for commercial distribution by the labeler on record.
Alternative or additional identifiers – Indicates if the device is exempt from Direct Marking (DM) requirements under 21 CFR 801.45, if the DM DI Number is different than the Primary DI Number and what the secondary DI, previous DI, or package DI is.
Customer contact – Phone and email address of a customer service contact is required so patients and consumers can ask any device-related questions.
Premarket – Determines if the device is exempt from premarket submission or the FDA premarket submission number.
FDA product code and listing – Both the FDA product code (a categorization for devices) and Product Code Name (associated with the three-letter Product Code) are required in addition to the listing number, which is assigned by FDA during registration and listing of all devices in commercial distribution, regardless of pre-market authorization requirements.
Storage, handling and sterilization – Identifies device storage and handling requirements (such as temperature) and sterilization specifications including, packaged as sterile or requires sterilization prior to use.
Wants to Read More About: barcode services
Preparing for Submission to GUDID
A critical prerequisite step for submitting device data to the FDA begins with establishing a GUDID account. To do so you will need to decide how many GUDID accounts are needed. Each account is tied to a separate Labeler Organization, such as corporate headquarters. How large is the organizational structure? If it is small to medium-sized, usually only one account is necessary. What about larger companies? Bigger companies may request a single account for all products OR create multiple accounts for differing portfolio segments.
Both Labeler Organizations and Labelers must be sourced in the account application with their Dun & Bradstreet DUNS number. Note, while application for a DUNS number is free, the process may take up to 30 business days.
How to Submit
Organizations must determine how they want to submit data through one of two methods: manual data entry or Health Level 7 (HL7) Structured Product Labeling (SPL). Some considerations include:
Does the company have a small number of records to submit? If so, they may want to enter data into the database manually through the free web interface, although the cost of staff time and expertise needed to enter and validate data should be considered regardless of device amount.
Does the company have a high volume of submissions? If so, they will likely need to submit data in an SPL format, requiring data conversion for submission-ready SPL files (through the FDA Electronic Submissions Gateway (ESG)). In this case, companies can purchase/build software or work with third-party providers, such as Reed Tech, for expertise in both validating and submitting data.
| GUDID Web Application Overview | HL7 SPL File Submission Overview |
| Ideal method for fewer records needed for GUDID submission | Ideal method for several records needed for GUDID submission |
| Requires manual data entry | Allows bulk submission and requires technical knowledge |
Once companies determine their submission method, they must identify GUDID account personnel and request a GUDID account from the FDA. If they want to submit data in an SPL format, the SPL submissions need to be tested (in a separate test account provided by FDA) to ensure submissions flowing through the ESG are successfully imported.
Are You Affected by the Class I Deadline?
Class I and unclassified medical devices required to be labeled with a UDI code must submit product data to the FDA GUDID by September 24, 2022. Medical device companies must first determine if their products are affected by this deadline. Do any of the following conditions apply to you?
- Class I device is distributed to professional healthcare facilities (hospitals, clinics, physician offices, etc.)
- Class I device is intended only for use by healthcare professionals
- Class I device is reused on different patients and reprocessed using high-level disinfection and/or sterilization
- Class I device requires 510(k)
Note there is a Class I GUDID Submission Exception for certain Class I devices that are 510(k) exempt and only sold over the counter to consumers.
Some examples for product types requiring UDI in a professional use, such as a hospital, include hospital beds, stethoscopes, dental instruments and equipment (chairs). Certain consumer use products (such as sunglasses, pill crushers, electronic toothbrushes) require UDI but not GUDID submission. Products sold in both professional settings and over the counter require UDI and include items such as band aids, first aid kids, and dental floss.
Upon determining if a product falls within the Class I submission regulation, FDA compliance for Class I devices includes:
Labeling – The UDI must be on the device product and package labels in a human-readable plain-text and Automatic Id and Data Capture (AIDC) technology, with the date format listing in YYYY-MM-DD (except AIDC date must use Issuing Agency date format)
Direct marking – Multiple use or reprocessed devices must have the UDI permanently marked
Submission to GUIDID – DI & device attributes (57 total) must be submitted to GUDID (through public portal, AccessGUDID)
Reporting and retention – UDI must be included in Annual Reports, DHR, complaints, MDR, recalls, service, tracking, post market surveillance, in addition to electronic record compliance (as part of 21 CFR 11) and a 3-year record retention (even after device is off of the market)
How to Comply with US FDA Class I, GUDID Requirements
A common question I hear from our customers, especially those with Class I medical device products, is “where do we begin?” Here is an implementation summary for you to reference as the Class I submission deadline quickly approaches:
UDI process prep – Determine who in your organization is responsible for UDI submissions and create a UDI governance team. Also identify FDA UDI requirements for your products, evaluate your situation and prepare the UDI environment.
Data prep – Understand what data elements are required, collect source GUDID data as well as normalize and validate data. If needed, you can work with a vendor, such as Reed Tech, to help with this step.
GUDID system and submission – Evaluate, select, and implement a GUDID solution, create FDA GUDID & ESG accounts, submit GUDID data to FDA and verify GUDID submission and publication.
Labeling system – Prepare labeling environment and test labels.
UDI operation – Start production and maintain data and systems, in addition to preparing for international UDI requirements for various health authorities around the globe.

Business
How Businesses Can Foster Safe and Inclusive Workplaces

What Is a Safe and Inclusive Workplace?
Creating a safe and inclusive workplace is an ongoing commitment that requires intentional policies, continuous education, and accountable leadership. When organizations prioritize respect, equity, and psychological safety, employees are more engaged, productive, and innovative. Integrating wellness resources, such as opportunities to learn about nutrition Clarkston, MI, further supports employees’ overall well-being and reinforces a culture of care. By measuring progress, addressing challenges proactively, and embedding inclusion into everyday practices, companies comply with legal standards and cultivate a culture where everyone can thrive—driving long-term success for both people and the business.
Why Company Culture Matters
A company’s culture is the collective personality that shapes how people interact, collaborate, and solve problems. Company culture is rooted in visible behaviors, day-to-day operations, written values, and the subtle ways leaders and colleagues interact. An inclusive culture championed by management sets a positive tone and signals to staff that respect, equity, and fairness are non-negotiable values.
A positive culture makes it easier for people of all backgrounds and abilities to feel seen and heard. Leaders who uphold these principles ensure that everyone, from interns to executives, understands what inclusion looks like in everyday situations.

Building Effective Policies
Robust, visible policies underpin successful, safe, and inclusive workplaces. These must extend beyond anti-discrimination language to address the diverse needs of employees, such as accommodations for religious practices, disabilities, gender identity, and parental responsibilities. Policies should be developed with input from employees and subject-matter experts to ensure they are practical, relevant, and current.
- Clearly define what safety and inclusion mean for your business and sector.
- Create straightforward reporting procedures, ensuring every team member knows how and where to report issues.
- Outline fair investigation processes and consequences for policy violations to reinforce accountability.
- Review all policies annually and communicate updates regularly to reinforce expectations.
To foster trust, ensure that all documents are easily accessible and comprehensible, and provide training that enables policies to be effectively implemented in real-life situations.
Training and Education Programs
Continuous education is essential for changing mindsets and behaviors. Training efforts focusing on diversity, equity, and inclusion should go beyond awareness-raising, helping team members understand how to call out exclusionary practices and become effective allies. Regular workshops, simulations, and discussions of real-world scenarios ensure that information sticks and leads to lasting change.
Leadership and Accountability
Leadership’s role is critical in setting expectations and modeling desired behaviors. Senior management and supervisors must be trained to recognize unconscious biases, address issues swiftly, and celebrate team successes related to inclusion. Regular performance reviews that assess leadership’s inclusivity ensure ongoing commitment.
Transparency in how issues are handled and shared success stories fosters a sense of collective responsibility. When accountability is embedded at all levels—from frontline workers to executives—employees understand that safe and inclusive behavior is a non-negotiable standard.
Measuring Progress and Success
Determining if policies and programs are effective starts with clear targets and ongoing evaluation. Many organizations use employee surveys, pulse checks, or third-party audits to collect meaningful qualitative and quantitative feedback. These insights reveal whether staff feel safe and valued and can surface areas requiring further improvement.
Regularly sharing progress fosters transparency and trust, making clear that employee voices directly shape company priorities.
Common Challenges and Solutions
Roadblocks may arise during efforts to build a safe and inclusive environment. Resistance to change, a lack of resources, or legacy behaviors can create setbacks. Overcoming these challenges requires intentional communication, dedicated resources, and ongoing reinforcement of the business case for inclusion.
- Resistance to Change: Address skepticism with transparent communication and real-life workplace benefits.
- Unconscious Bias: Combat through tailored training sessions and reflective leadership assessments.
- Resource Constraints: Start small by celebrating early wins and leveraging free external toolkits and resources.
Keeping communication channels open and fostering psychological safety helps sustain engagement and momentum, helping organizations stay adaptable in a changing landscape.
Final Thoughts
Creating a safe and inclusive workplace is an ongoing commitment that requires intentional policies, continuous education, and accountable leadership. When organizations prioritize respect, equity, and psychological safety, employees are more engaged, productive, and innovative. By measuring progress, addressing challenges proactively, and embedding inclusion into everyday practices, companies comply with legal standards and cultivate a culture where everyone can thrive—driving long-term success for both people and the business.
Business
5 Success Stories from Leading Logistics Marketing Agencies

In today’s rapidly evolving marketplace, logistics marketing agencies play a crucial role in bridging the gap between supply chain efficiency and customer engagement. These specialized firms leverage a deep understanding of the logistics sector, utilizing innovative strategies to help companies maximize their reach and drive growth. Here, with the increasing complexity of global trade, the demand for adept marketing tailored specifically to the logistics industry has surged here today’s rapidly evolving marketplace, logistics marketing agencies play a crucial role in bridging the gap between supply chain efficiency and customer engagement. These specialized firms leverage a deep understanding of the logistics sector, utilizing innovative strategies to help companies maximize their reach and drive growth. Here, with the increasing complexity of global trade, the demand for adept marketing tailored specifically to the logistics industry has surged.
These agencies are not just about promoting services; they are data-driven, crafting campaigns that resonate with target audiences while enhancing brand visibility. From search engine optimization (SEO) to targeted digital advertising, they employ a range of techniques to ensure that logistics companies stand out in a crowded marketplace.
As we delve into the success stories of various logistics marketing agencies, we will uncover how they have transformed their clients’ operations, enhanced their online presence, and ultimately driven significant business outcomes. By showcasing these achievements, we aim to highlight the invaluable role logistics marketing agencies play in the modern business landscape, making them indispensable partners for companies striving to thrive in this competitive environment.
Importance of Marketing in the Logistics Industry
In the increasingly competitive logistics industry, effective marketing is not just a luxury — it’s a necessity. The nuances of this sector demand a strategic approach that combines traditional marketing techniques with advanced digital strategies. As logistics firms strive to differentiate themselves, marketing becomes the driving force behind brand recognition and customer loyalty.
One of the primary roles of marketing in logistics is to educate potential clients about the complexities and benefits of various services. With rapid technological advancements and shifting consumer expectations, agencies that effectively communicate their value propositions can capture market share and establish trust.
Moreover, targeted marketing campaigns help logistics companies to reach niche markets and tailor their offerings accordingly. By leveraging data analytics, agencies can identify customer pain points and provide solutions that resonate with specific audiences.
Finally, a strong marketing presence enhances a company’s reputation in an industry often plagued by misconceptions. By showcasing success stories and transparent operations, logistics firms can build credibility and foster long-term relationships. In essence, marketing is not merely an add-on; it’s the backbone of growth and sustainability in the logistics landscape.
Overview of Success Stories
In the ever-evolving landscape of logistics, marketing agencies have emerged as pivotal players, transforming how companies connect with their audiences. These success stories highlight innovative strategies that have led to remarkable growth and brand recognition, with the specific agencies involved remaining undisclosed due to NDA agreements.
One standout example involves a mid-sized freight company that, with the help of a specialized marketing agency, revamped its digital presence. By implementing a targeted SEO strategy and launching engaging content campaigns, they saw a 150% increase in organic traffic within six months.
Another success story features a logistics startup that harnessed the power of social media advertising. Through compelling visuals and customer testimonials crafted by their marketing partner, they not only amplified their brand visibility but also achieved a 300% increase in lead generation within a year.
Additionally, a renowned global shipping firm collaborated with a marketing agency to enhance customer engagement through personalized email campaigns. The result? An impressive 45% boost in customer retention rates.
These narratives illustrate the profound impact that strategic marketing initiatives can have on logistics businesses, driving growth, fostering innovation, and ultimately reshaping the industry landscape.
Case Study 1: Innovative Strategies from Agency A
Agency A has redefined success in the logistics marketing arena through a blend of innovative strategies that cater specifically to the unique challenges of the industry. One standout initiative involved a comprehensive digital transformation project for a mid-sized freight company struggling with visibility and market penetration.
Recognizing the importance of data-driven decisions, Agency A implemented advanced analytics tools to assess customer behavior and identify key pain points. This insight laid the groundwork for a targeted content marketing strategy that included informative blogs, engaging videos, and interactive webinars, tailored to educate potential clients on industry trends and best practices.
To complement this content strategy, Agency A leveraged social media platforms, creating a robust online presence that allowed the client to connect directly with their target audience. A series of compelling case studies showcasing successful logistics solutions further established the client as a thought leader in the space.
The results were impressive: within six months, website traffic surged by 150%, and lead generation doubled. Agency A’s innovative approach not only improved brand visibility but also fostered lasting relationships, proving that thoughtful marketing can transform logistics businesses in a competitive landscape.
Case Study 2: Transformative Campaigns by Agency B
Agency B, a trailblazer in logistics marketing, recently executed a transformative campaign for a mid-sized freight company struggling to stand out in a saturated market. The agency initiated a thorough market analysis, identifying key pain points within the target audience — specifically, the need for transparency and reliability during shipment processes.
With insights in hand, Agency B crafted a multi-platform strategy that prominently featured customer testimonials and real-time tracking features. They launched an engaging social media campaign showcasing behind-the-scenes operations, which humanized the brand and built trust with potential clients. The campaign’s centerpiece was an interactive website redesign that emphasized user experience, allowing clients to easily access shipment status and logistics updates.
The results were remarkable. Within six months, the freight company saw a 40% increase in website traffic and a 25% boost in lead generation. Most impressively, customer retention rates improved significantly, as clients felt more connected and informed throughout the shipping journey. Agency B’s innovative approach not only transformed the freight company’s brand image but also set a new standard for customer engagement in the logistics sector, demonstrating the power of targeted, transparent marketing.
Case Study 3: Data-Driven Success from Agency C
- Increased Website Traffic: A 150% surge in monthly visitors due to optimized SEO and strategic content marketing.
- Lead Generation: The campaign generated a 200% increase in qualified leads within just three months.
- Conversion Rates: The brokerage saw a 35% rise in conversion rates, directly linking data-driven personalization to successful customer engagement.
- Cost Efficiency: By reallocating budget to high-performing channels identified through analytics, the client reduced their customer acquisition cost by 25%.
- This case exemplifies how Agency C’s data-driven methodologies not only foster growth but also empower logistics companies to make informed decisions that drive long-term success.
Case Study 4: Creative Branding by Agency D
Agency D took a bold step in redefining its client’s brand identity, which was struggling to resonate in an increasingly competitive logistics market. The agency embarked on a comprehensive rebranding campaign that highlighted the company’s commitment to sustainability and innovation.
Through meticulous market research, Agency D discovered that their client’s target audience was particularly passionate about eco-friendly practices. Leveraging this insight, they crafted a striking new logo and a vibrant visual identity that incorporated green elements and modern typography. The campaign’s centerpiece was a digital storytelling initiative, showcasing the logistics company’s journey toward sustainability through engaging video content and infographics.
This multifaceted approach not only revamped the brand’s image but also created a community around its values. The result? A remarkable 40% increase in brand engagement on social media platforms and a 30% rise in customer inquiries within just six months. Agency D’s creative branding efforts not only repositioned the client as a leader in sustainable logistics but also fostered lasting connections with environmentally-conscious consumers. This case highlights how innovative branding can transform perceptions and drive tangible results in the logistics sector.
Case Study 5: Technology Integration by Agency E
In an era where technology dictates market dynamics, Agency E has revolutionized logistics marketing through strategic technology integration. By leveraging advanced analytics and AI-driven insights, Agency E transformed a traditional freight brokerage into a tech-savvy logistics powerhouse.
The agency embarked on a comprehensive data-driven campaign that incorporated machine learning algorithms to predict shipping trends and optimize routes. This not only enhanced operational efficiency but also significantly reduced costs for their clients. By integrating a user-friendly digital platform, they provided real-time tracking capabilities, allowing customers to monitor their shipments with unparalleled transparency.
Additionally, Agency E implemented a customer relationship management (CRM) system tailored to the logistics sector, enabling personalized communication and targeted marketing strategies. This integration led to a 40% increase in customer engagement and a dramatic rise in repeat business.
The success of this initiative is reflected in the agency’s remarkable 30% growth in revenue over just one year. By marrying logistics with cutting-edge technology, Agency E has set a benchmark for the industry, proving that innovation is key to thriving in a competitive landscape. Their story serves as an inspiration for other logistics marketing agencies looking to harness the power of technology for success.
Key Takeaways from the Success Stories

The success stories from leading logistics marketing agencies reveal several key takeaways that can inspire others in the industry. First and foremost, the importance of data-driven decision-making stands out. Agencies that leverage analytics to tailor their strategies consistently outperform those that rely on intuition alone. This data-centric approach not only enhances targeting but also improves ROI.
Additionally, effective storytelling plays a crucial role in connecting with clients. Agencies that have mastered the art of narrative can vividly convey their brand’s value proposition, fostering trust and engagement. This emotional connection often translates into long-term client relationships.
Moreover, the adaptability of these agencies in the face of market changes highlights the need for agility. Those that pivot quickly in response to evolving consumer demands or technological advancements are better positioned for sustained success.
Lastly, collaboration emerges as a vital theme. Successful agencies prioritize partnerships, whether with tech firms for innovative solutions or with clients to co-create marketing strategies. This collaborative spirit not only enriches campaigns but also ensures they resonate deeply with target audiences, securing loyalty in an increasingly competitive landscape.
Conclusion and Future Trends in Logistics Marketing
As we reflect on the remarkable success stories from leading logistics marketing agencies, it’s clear that innovation and adaptability have been the cornerstones of their achievements. These agencies have not only enhanced their clients’ visibility but also transformed traditional logistics marketing into a dynamic, data-driven endeavor.
Looking ahead, we can anticipate several key trends shaping the future of logistics marketing. First, the integration of artificial intelligence and machine learning will allow for hyper-personalized marketing strategies, enabling agencies to tailor campaigns that resonate deeply with target audiences. Additionally, the rise of sustainability in logistics will prompt marketers to emphasize eco-friendly practices, appealing to environmentally conscious consumers.
Furthermore, omnichannel marketing will become increasingly vital, as businesses strive to create seamless experiences across various platforms. This approach will require agencies to harness analytics to track customer interactions and preferences effectively.
Lastly, the growing significance of real-time tracking and transparency in logistics services will demand innovative storytelling techniques, allowing brands to showcase their efficiency and reliability. As these trends unfold, logistics marketing agencies are poised to lead the charge, driving growth and reinforcing the vital role of logistics in the global economy.
Business
Dead And Co Setlist What They Played At The Gorge Amphitheatre
Dead And Co Setlist What They Played At The Gorge Amphitheatre
Last weekend, Dead And Co Setlist descended upon the Gorge Amphitheatre in George, WA for their annual three-night run. As always, the boys delivered an amazing show, playing a mix of old favourites and new classics. If you weren’t able to make it out to the Gorge this year (or even if you did), here’s a look at the setlist from all three nights.
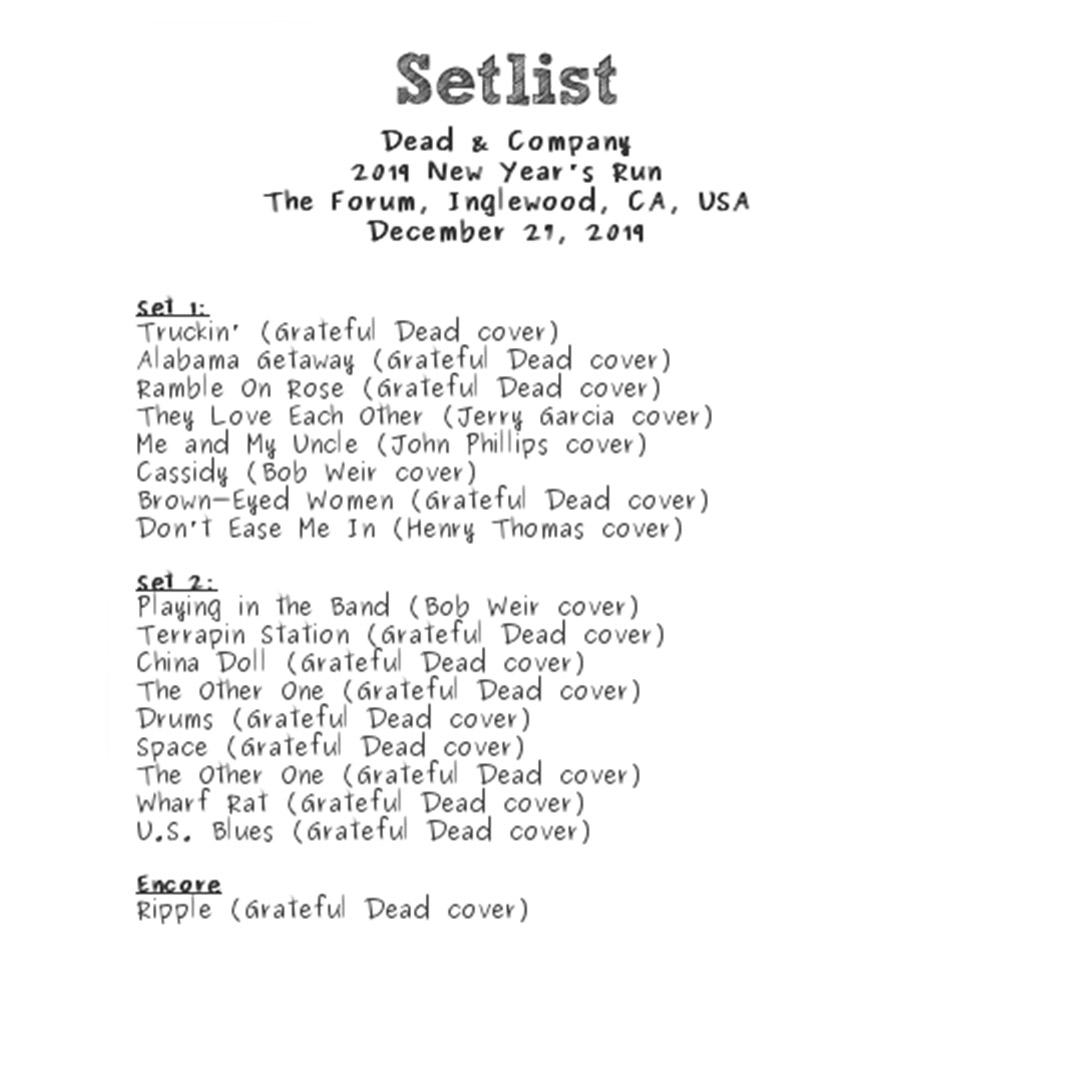
Dead & Company’s Setlist from their show at the Gorge Amphitheatre
Dead & Company’s Setlist from their show at the Gorge Amphitheatre:
1. “Bertha”
2. “Me and My Uncle”
3. “Ramble On Rose”
4. “When I Paint My Masterpiece”
5. “Althea”
6. “Cassidy”
7. “Don’t Ease Me In”
8. “Cold Rain and Snow”
9. “The Music Never Stopped”
10. “Deal”
11. “‘Til the Morning Comes”
Set 2:
12. “Help on the Way” >
13. “Slipknot!” >
14. “Franklin’s Tower”
15. Playin’ in the Band >
16. Uncle John’s Band >
17. Playin’ Reprise
18. Dark Star >
19 Drumz/Space > 20 Dark Star Reprise 21 Stella Blue 22 One More Saturday Night Encore: 23 Ripple
Highlights of the show
The show started with “Bertha” and included several other Grateful Dead classics like “Sugaree,” “Jack Straw,” “Deal,” and “Tennessee Jed.” The set also featured a couple of covers, including The Beatles’ “Dear Prudence” and Bob Dylan’s “All Along the Watchtower.”
The second set was even better, starting with an incredible “Fire on the Mountain” and a heart-stopping “Scarlet Begonias.” Other highlights from the second set include “Playin’ in the Band,” the classic jam vehicle, as well as a personal favorite, “Drums” > “Space.” The show ended with a beautiful performance of Jerry Garcia’s solo work, “Stella Blue.”
Final thoughts
This show was a celebration of life itself. After such a trying year, it is important to reflect on what we have accomplished and how lucky we are. I hope that everyone takes some time to think about this idea this month as we begin 2021.
What fans are saying about the show
The Grateful Dead have been one of the most influential bands in music history, and their fans are some of the most dedicated in the world. So when Dead & Company, a new band featuring original members Bob Weir and Mickey Hart, announced their first tour, fans were eager to see what they would do.
The band’s debut show at the Gorge Amphitheatre was a resounding success, with fans raving about the setlist. Highlights included renditions of classic Dead songs like “Bertha,” “Sugaree,” and “Althea,” as well as newer tunes like “Fire on the Mountain” and “Iko Iko.” The band also paid tribute to late Grateful Dead leader Jerry Garcia with a moving performance of “Black Muddy River.”
It’s clear that Dead & Company are already making waves with fans old and new. If their debut show is any indication, this is a band that is here to stay.
Setlist video
The setlist for Dead and Company’s show at the Gorge Amphitheatre was:
“Iko Iko”
“Bertha”
“New Speedway Boogie”
“Wharf Rat”
“He’s Gone”
“Fire on the Mountain”
“Drums/Space”
“The Other One”
“Stella Blue”
“Sugaree
Encore:
Touch of Grey
Upcoming Dead & Company tour dates
The wait is finally over! After a long hiatus, Dead & Company are back on tour and will be making their way to the Gorge Amphitheatre this summer. The tour kicks off on June 10th in Boulder, Colorado and will make its way across the country before concluding on July 6th in Boston, Massachusetts. Below is a list of all the upcoming tour dates:
Date June 10 – Boulder, CO @ Folsom Field
On June 13 – Albuquerque, NM @ Isleta Amphitheatre
June 15 – Phoenix, AZ @ Ak-Chin Pavilion
Check June 17 – Los Angeles, CA @ Hollywood Bowl
View June 20 – Mountain View, CA @ Shoreline Amphitheatre
June 22 – San Diego, CA @ Mattress Firm Amphitheatre
July 5 – Saratoga Springs, NY @ Saratoga Performing Arts Center
July 6 – Boston, MA @ Fenway Park

 Travel2 years ago
Travel2 years agoPractical And Essential Car Interior Accessories To Add Comfort And Convenience To Your Drive

 Business2 years ago
Business2 years agoTop Reasons Why you Need to Consider Outsourcing Real Estate Photo Editing

 Business2 years ago
Business2 years agoDead And Co Setlist What They Played At The Gorge Amphitheatre

 Featured2 years ago
Featured2 years agoHow to Make a Sports Career in India

 Health2 years ago
Health2 years agoGarlic Is The Best Vegetable To Treat Heart Problems

 Sports2 years ago
Sports2 years agoHow to watch the ETSU game -What are the benefits of watching the ETSU game?
 Health2 years ago
Health2 years ago5 Reasons to Choose Turkey for Dental Treatments

 Travel2 years ago
Travel2 years agoSpectacular Hot Air Balloon Rides in Goa